I decided to do some more research into the period tableland the elements themselves. This is so I can understand fully what they actually are and how they are made up before I go further into my project.
What is an element?
A Chemical Element is a pure substance consisting only of atoms that all have the same numbers of protons in their nuclei. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical.
I then waned to see visually what an element is and then found this helpful diagram.

From looking at this diagram I now understand that there are atoms. I now need to know what an atom is and what makes atoms different to each other. So I took to the Internet again to find some more information on an atom
What is an atom?
An atom is the smallest unit of ordinary matter that forms a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small.
Atom Diagrams
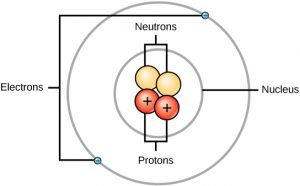
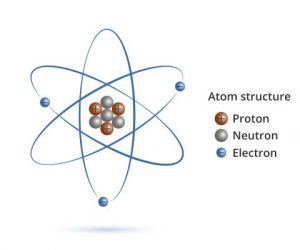
From looking closely at these atoms I now understand that within every atom there are protons, neutrons and electrons. That the number of each of these parts of an atom changes with every element. The nucleus is the centre of the atom, where the protons and neutrons are held. The electrons surround this.
The correlation between the elements and atoms
How do atoms relate to the periodic table?
‘The properties of an atom relate directly to the number of electrons in various orbitals, and the periodic table is much like a road map among those orbitals such that chemical properties can be deduced by the position of an element on the table.’ – Lumen Learning
Elements can be identified by their atomic number and mass number.

Above is a great diagram I found that explains the information on the elements easily, is shows the atomic number, name, symbol and relative atomic mass of Hydrogen. All of this information is on each element on the periodic table, so it is really helpful to have each part explained. This demo I found last colour codes the table into the non -metals, noble gases, other metals etc. This is very helpful and a great tool for researching the elements I want to use.
What did I learn?
I now know how each element has different make up, had different atomic numbers, and this is how they are placed on the periodic table. I also am aware that each element on the table has numbers and symbols that now make sense to me. I also learned what the structure of an atom looks like. This was something I. had forgot form school but am now aware of again, I know the nucleus is in the centre and electrons surround it.
Why do this research into the breakdown?
I wanted to learn what an element actually was and how they related back to atoms etc. I also wanted to get a clearer understanding as to why elements are in that order on the table and what the numbers mean. I felt this was very helpful in my development because now I know this, I can move forward into thinking about how this would be including into my book for kids.